Lucullus – Welcome to Bioprocessing 4.0
The biotechnology industry has a profound need for modernisation, indeed, with the current COVID-19 pandemic, biopharmaceutical manufacturers are under more pressure than ever before to produce therapeutics in high volume and shorter-time frames without jeopardising patient safety. As grim as the world seems in the midst of a global pandemic, the future of bioprocess development and manufacture is both hopeful and profoundly exciting.

Industry 4.0 is a term used to describe a 4th generation industrial revolution, where manufacturing is fully digitised, centralised, and automated. Since its inception in industries including Oil & Gas and Logistics, there has been improved interconnection across supply chains and manufacturing unit operations for a number of years. The bioprocessing 4.0 model resembles a fully digitised and end-end connected process, with equipment across the manufacturing stream along with the necessary analytics; connected, controlled, and automated.
Lucullus provides an insight into the future of bioprocess development and manufacturing. Not only does the software facilitate a streamlined and more efficient use of time and resources, but exponentially simplifies traceability and data integrity by facilitating a centralised and CFR 21 Part 11 compliant audit trail attributable to an individual user. Consequently, Lucullus PIMS is already beginning to see use in everything from research laboratories to GMP manufacturing facilities.
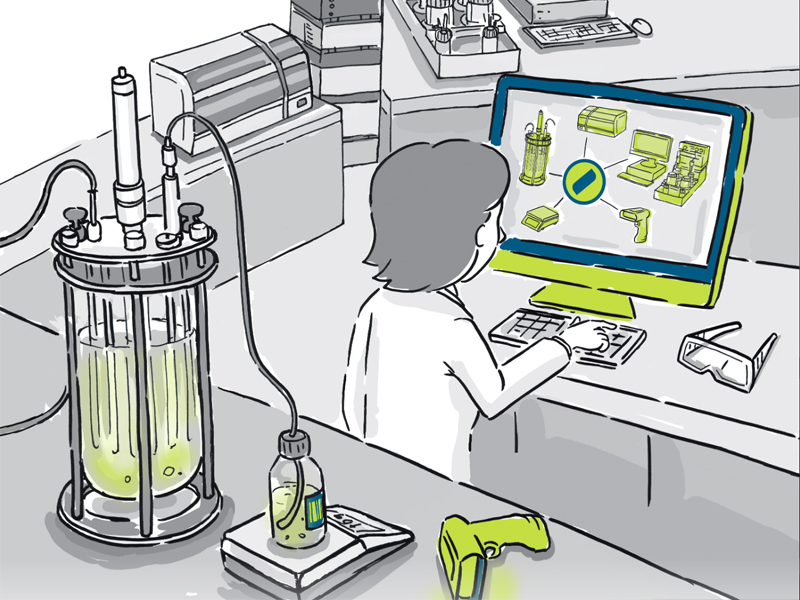
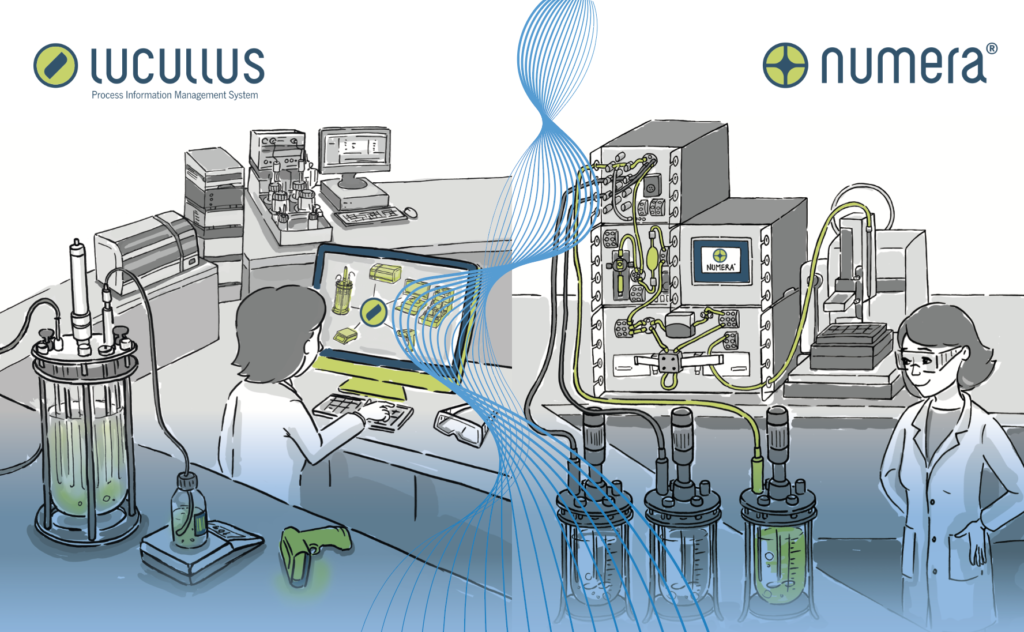
The Lucullus Process Integration Management System (PIMS) by Securecell in Zurich, is one of the first products on the market where the vision of bioprocess 4.0 begins to become a reality. Using a series of servers that support open platform communication standards (OPC DA/UA) and equipment specific drivers, Lucullus is able to digitise and link almost any piece of process equipment and their peripherals into a network that centralises and harmonises monitoring and control of bioprocessing to a single user interface. Advanced process controls allows commands to be implemented in real-time from one location, facilitating quick responses to any changes in conditions or requirements with any piece of equipment throughout the manufacturing stream. Furthermore, the scalable architecture allows an unlimited number of parallel processes to be orchestrated from a single client. This potentially revolutionises the speed at which process development work can be performed, meaning processes can be optimised and made ready to enter GMP manufacture in a much faster and more efficient time frame.
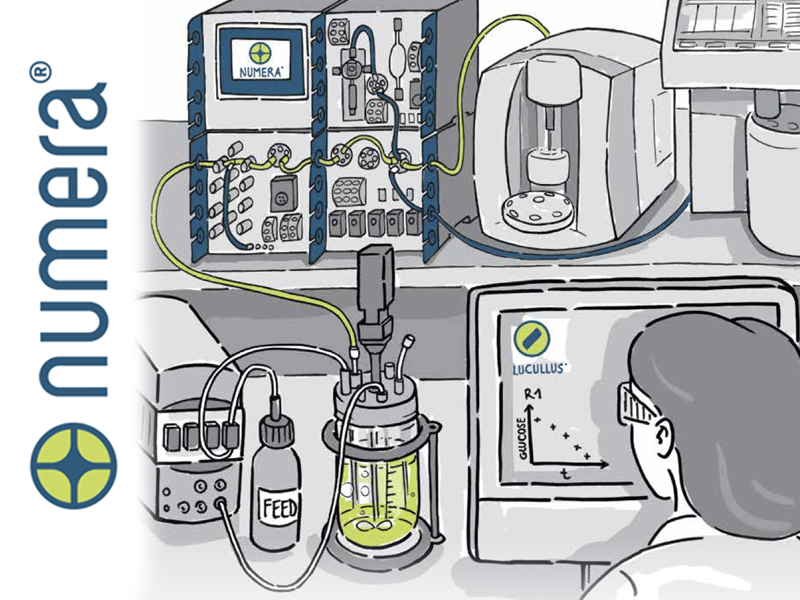
Peripheral equipment can also be integrated into Lucullus, for example an Aber Futura probe in a bioreactor can feed biomass data into the system, which reports seamlessly to the user through the interface. Additionally, previously physical documentation heavy tasks can be digitised and managed through the software. Media preparation, for example, can be facilitated through use of a media recipe database assisted by a barcode labelling and user guided lot creation tool. This not only facilitates traceability of all relevant user actions/interactions, but can also feed back into the supply chain and allow for an overview of when raw material stocks are becoming low or approaching expiry dates.
Sample data management can also be fully digitised, with Lucullus acting as a bridge between at-line and offline-line analytics. Sample plans can be scheduled as well as labelled with barcodes for seamless identification and audit, and previously isolated analytical equipment can record automatically into the network. Most significantly of all, using Securecell’s Numera analyser, all process analytics can be fully automated, with 24/7 on-line analysis performed on the stream without the time delay and infrequency of manual, offline recording. Furthermore, sampling consistency performed by a fully automated system ensures a highly reliable and repeatable process.
Biopharmaceutical manufacturing involves use of many categories of equipment which is often bespoke to the needs of that particular organisation and involves use of proprietary interfaces which output data in many different formats depending on brand etc. Additionally, some tasks such as media preparation and offline analytics often necessitate the generation of physical paper documents which must be carefully transcribed and stored. Consequently, process data is disparate and requires manual and time consuming organisation in order to report meaningful results.
Considering that other industries already demonstrate the effectiveness and benefits enjoyed through an integrated manufacturing process, as well as the public perception of biotechnology as being at the cutting edge (at least scientifically) of technology, it seems surprising at first that bioprocess modernisation has not been faster. However, the industry is heavily regulated, with concerns over how such a game-changing technology would impact regulatory compliance including Good Manufacturing Process (cGMP) where implementing changes to an already established process is perceived at least as being extremely complicated. But, with the development of game-changing technologies such as Lucullus, how long can it be until biopharmaceutical manufacturers realise that the gains far outweigh the costs?
Article by:
Sean Doran
Technical Sales Specialist
E. sdoran@bioprocess-eng.co.uk